Neural sensitivity to voice
Description
Complementary neuroimaging (fMRI, EEG, MEG) and interference (TMS) techniques are used to detail the spatiotemporal dynamics of cerebral voice processing in normal adults, and the changes associated with development and ageing.
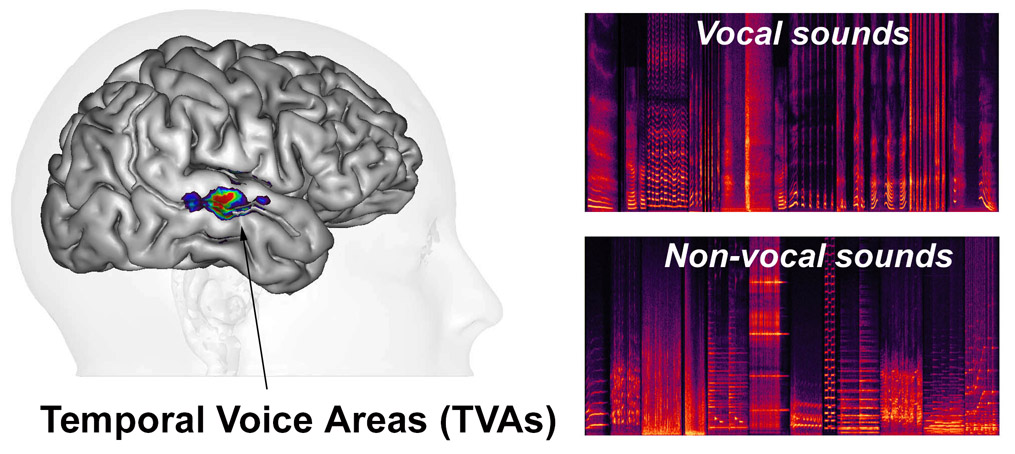
Secondary areas of human auditory cortex along the superior temporal gyrus (STG) and sulcus (STS) both anterior and posterior to primary auditory cortex contain temporal voice areas (TVAs) (Belin et al, 2000, 2002; Pernet et al , 2015 ) that show greater fMRI signal in response to vocal sounds—whether they contain speech or not—than to other categories of non-vocal sounds such as environmental sounds, amplitude-modulated noise, etc. (Agus, 2017) or to hetero-specific vocalizations (HVs) (Fecteau et al, 2004 ). They can be considered as the auditory analogues of the “face areas” or “face patches” of visual cortex.
The TVAs are quite variable between individuals in exact anatomical location but a cluster analysis of voice-sensitivity peaks in several hundred subjects (Pernet et al, 2015) suggests an organization in three “voice patches” along STG/STS bilaterally (TVAa, TVAm, TVAp; Figure).
Voice processing also engages cerebral areas outside of auditory cortex, including several prefrontal areas (particularly in the inferior frontal gyrus bilaterally (Fecteauet al, 2005; Pernet et al, 2015).
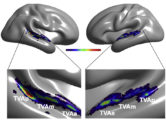 Figure – The human Temporal Voice Areas (TVAs) are organized in three rostro-caudal “voice patches” along the STS and STG in the temporal lobe of each hemisphere with greater fMRI response to vocal vs. non-vocal sounds (Pernet et al, 2015).
Figure – The human Temporal Voice Areas (TVAs) are organized in three rostro-caudal “voice patches” along the STS and STG in the temporal lobe of each hemisphere with greater fMRI response to vocal vs. non-vocal sounds (Pernet et al, 2015).
The anatomo-functional organization of the TVAs is still poorly understood. Their causal link with voice processing has been established in a single study so far: transiently interfering with neuronal activity in the right TVAm via transcranial magnetic stimulation (TMS) interferes with performance at a voice detection task but not at a more general auditory task (Bestelmeyer et al, 2011 ).
One outstanding issue is that of nature vs. nurture: Is voice-selectivity in the TVAs the result of evolutionary tuned innate mechanisms or does it reflect the extensive experience during development and adulthood with this ecologically crucial sound category? No convincing answer to that question has been provided so far, perhaps because long-term manipulation of human listeners’ auditory environment is hard to perform.
Collaborators
Robert Zatorre, Cyril Pernet, Marie-Helene Grosbras, Jingting Zhang, Patricia Bestelmeyer, …
Relevant Publications
Agus, T.R., Paquette, S., Suied, C., Pressnitzer, D., Belin, P. (2017) Voice selectivity in the temporal voice area despite matched low-level acoustic cues. Scientific Reports Sep 14;7(1):11526. PDF
Bodin, C., Takerkart, S., Belin, P., Coulon, O. (2017) Anatomo-functional correspondence in the superior temporal sulcus. Brain Structure and Function PDF
Aglieri, V., Watson, R., Pernet, C., Latinus, M., Garrido, L., and Belin, P. (2016). The Glasgow Voice Memory Test: Assessing the ability to memorize and recognize unfamiliar voices. Behavior research methods. PDF
Pernet, C.R., McAleer, P., Latinus, M., Gorgolewski, K.J., Charest, I., Bestelmeyer, P.E., Watson, R.H., Fleming, D., Crabbe, F., Valdes-Sosa, M., and Belin, P. (2015). The human voice areas: Spatial organization and inter-individual variability in temporal and extra-temporal cortices. NeuroImage 119, 164-174. PDF
Yovel, G., and Belin, P. (2013). A unified coding strategy for processing faces and voices. Trends in cognitive sciences 17, 263-271. PDF
Capilla, A., Belin, P., and Gross, J. (2013). The early spatio-temporal correlates and task independence of cerebral voice processing studied with MEG. Cerebral cortex 23, 1388-1395. PDF
Belin, P., Zatorre, R.J., Lafaille, P., Ahad, P., and Pike, B. (2000). Voice-selective areas in human auditory cortex. Nature 403, 309-312. PDF





















